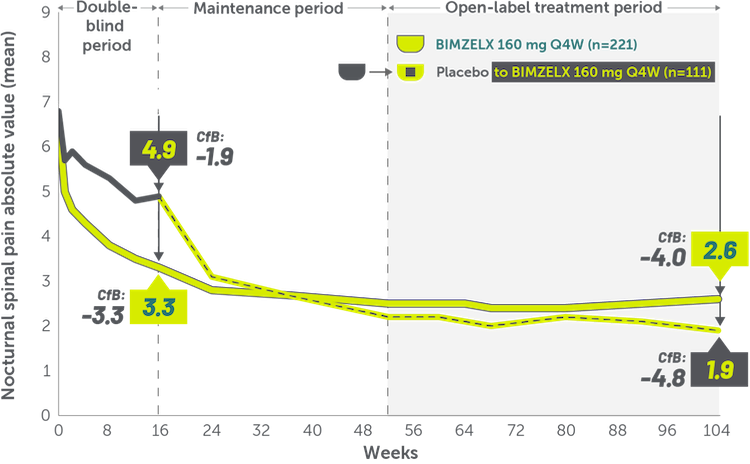
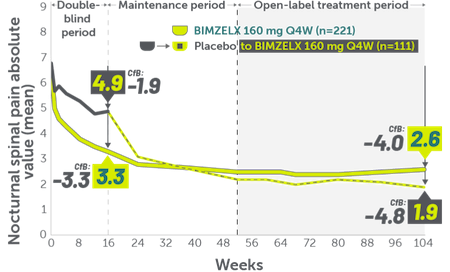
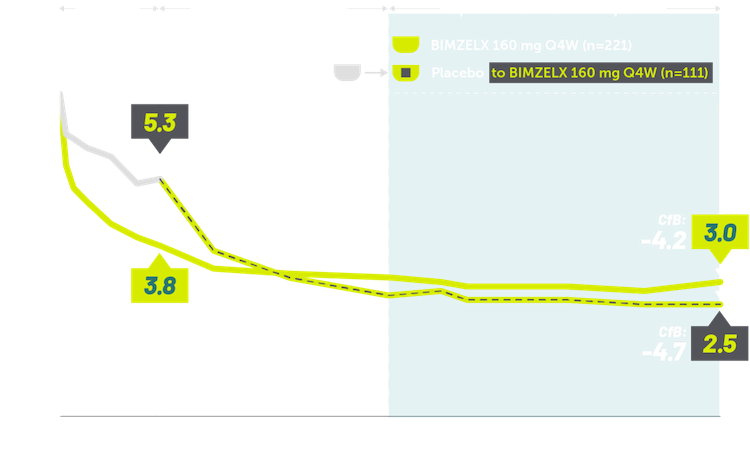
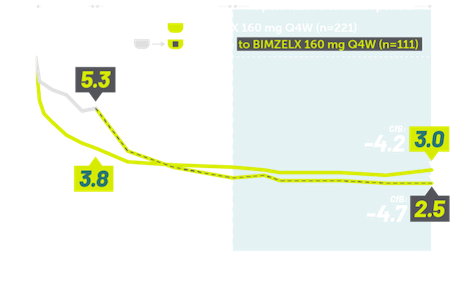
INDICATIONS
BIMZELX is indicated for the treatment of adults with active psoriatic arthritis, active non-radiographic axial spondyloarthritis with objective signs of inflammation, active ankylosing spondylitis, moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy, and moderate-to-severe hidradenitis suppurativa.
IMPORTANT SAFETY INFORMATION
Suicidal Ideation and Behavior
BIMZELX may increase the risk of suicidal ideation and behavior (SI/B). A causal association between treatment with BIMZELX and increased risk of SI/B has not been definitively established. Prescribers should weigh the potential risks and benefits before using BIMZELX in patients with a history of severe depression or SI/B. Advise monitoring for the emergence or worsening of depression, suicidal ideation, or other mood changes. If such changes occur, instruct to promptly seek medical attention, refer to a mental health professional as appropriate, and re-evaluate the risks and benefits of continuing treatment.
Infections
BIMZELX may increase the risk of infections, including serious infections. Do not initiate treatment with BIMZELX in patients with any clinically important active infection until the infection resolves or is adequately treated. In patients with a chronic infection or a history of recurrent infection, consider the risks and benefits prior to prescribing BIMZELX. Instruct patients to seek medical advice if signs or symptoms suggestive of clinically important infection occur. If a patient develops such an infection or is not responding to standard therapy, monitor the patient closely and do not administer BIMZELX until the infection resolves.
Tuberculosis
Evaluate patients for tuberculosis (TB) infection prior to initiating treatment with BIMZELX. Avoid the use of BIMZELX in patients with active TB infection. Initiate treatment of latent TB prior to administering BIMZELX. Consider anti-TB therapy prior to initiation of BIMZELX in patients with a past history of latent or active TB in whom an adequate course of treatment cannot be confirmed. Closely monitor patients for signs and symptoms of active TB during and after treatment.
Liver Biochemical Abnormalities
Elevated serum transaminases were reported in clinical trials with BIMZELX. Test liver enzymes, alkaline phosphatase, and bilirubin at baseline, periodically during treatment with BIMZELX, and according to routine patient management. If treatment-related increases in liver enzymes occur and drug-induced liver injury is suspected, interrupt BIMZELX until a diagnosis of liver injury is excluded. Permanently discontinue use of BIMZELX in patients with causally associated combined elevations of transaminases and bilirubin. Avoid use of BIMZELX in patients with acute liver disease or cirrhosis.
Inflammatory Bowel Disease
Cases of inflammatory bowel disease (IBD) have been reported in patients treated with IL-17 inhibitors, including BIMZELX. Avoid use of BIMZELX in patients with active IBD. During BIMZELX treatment, monitor patients for signs and symptoms of IBD and discontinue treatment if new onset or worsening of signs and symptoms occurs.
Immunizations
Prior to initiating therapy with BIMZELX, complete all age-appropriate vaccinations according to current immunization guidelines. Avoid the use of live vaccines in patients treated with BIMZELX.
MOST COMMON ADVERSE REACTIONS
Most common (≥1%) adverse reactions in plaque psoriasis and hidradenitis suppurativa include upper respiratory tract infections, oral candidiasis, headache, injection site reactions, tinea infections, gastroenteritis, herpes simplex infections, acne, folliculitis, other candida infections, and fatigue.
Most common (≥2%) adverse reactions in psoriatic arthritis include upper respiratory tract infections, oral candidiasis, headache, diarrhea, and urinary tract infections.
Most common (≥2%) adverse reactions in non-radiographic axial spondyloarthritis include upper respiratory tract infections, oral candidiasis, headache, diarrhea, cough, fatigue, musculoskeletal pain, myalgia, tonsillitis, transaminase increase, and urinary tract infections.
Most common (≥2%) adverse reactions in ankylosing spondylitis include upper respiratory tract infections, oral candidiasis, headache, diarrhea, injection site pain, rash, and vulvovaginal mycotic infection.
Please see the full Prescribing Information.
Most common (≥2%) adverse reactions in PsA, nr-axSpA, and AS include upper respiratory tract infections, oral candidiasis, headache, diarrhea, and urinary tract infections. Other most common (≥2%) adverse reactions specific to each indication include: urinary tract infections (PsA); cough, fatigue, musculoskeletal pain, myalgia, tonsillitis, transaminase increase, and urinary tract infections (nr-axSpA); injection site pain, rash, and vulvovaginal mycotic infections (AS).