RAPID, COMPLETE, AND MAINTAINED CLEARANCE1-5
A MAJORITY OF PATIENTS WERE COMPLETELY CLEAR AT WEEK 16
IGA 0/1 AT WEEK 16:
93% (BIMZELX Q4W) vs 1% (placebo) co-primary endpoint; P<0.00012
84% (BIMZELX Q4W) vs 5% (placebo) co-primary endpoint; P<0.00014
*Prespecified secondary endpoint adjusted for multiplicity.
Non-responder imputation.
IGA=Investigator’s Global Assessment; PASI=Psoriasis Area and Severity Index; Q4W=every 4 weeks.
*Prespecified secondary endpoint adjusted for multiplicity.
Non-responder imputation.
IGA=Investigator’s Global Assessment; PASI=Psoriasis Area and Severity Index; Q4W=every 4 weeks.
SKIN CLEARANCE YOU CAN SEE FROM THE VERY FIRST DOSE*
*From the very first dose as measured at Week 4; results were not immediate.
†Prespecified secondary endpoint adjusted for multiplicity.
‡Endpoints not adjusted for multiplicity. Nominal P value.
Non-responder imputation.
PASI=Psoriasis Area and Severity Index.
*From the very first dose as measured at Week 4; results were not immediate.
†Prespecified secondary endpoint adjusted for multiplicity.
‡Endpoints not adjusted for multiplicity. Nominal P value.
Non-responder imputation.
PASI=Psoriasis Area and Severity Index.
DURABLE SKIN CLEARANCE THROUGH 3 YEARS6
In the BE BRIGHT open-label extension study of phase 3 studies, 96.4% of patients who achieved PASI 90 at Week 16 maintained that level of clearance at 3 years.

In the BE BRIGHT open-label extension study of phase 3 studies, 82% of patients who achieved PASI 100 at Week 16 maintained that level of clearance at 3 years.

OLE LIMITATIONS: The open-label extension has limitations with a lack of comparator and the potential enrichment of the patient population.
Efficacy computed using modified NRI.
NRI=non-responder imputation; OLE=open-label extension; PASI=Psoriasis Area and Severity Index; Q4W=every 4 weeks;
Q8W=every 8 weeks.
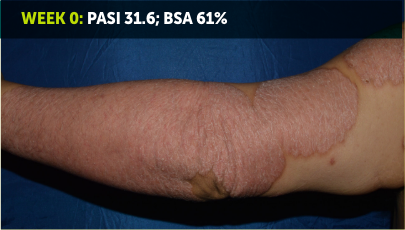
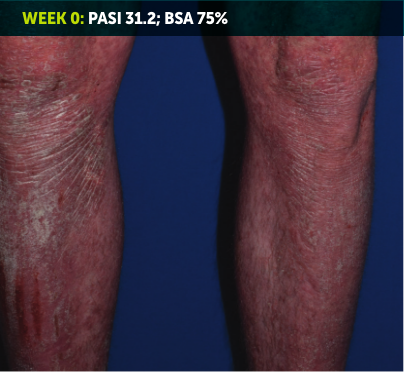
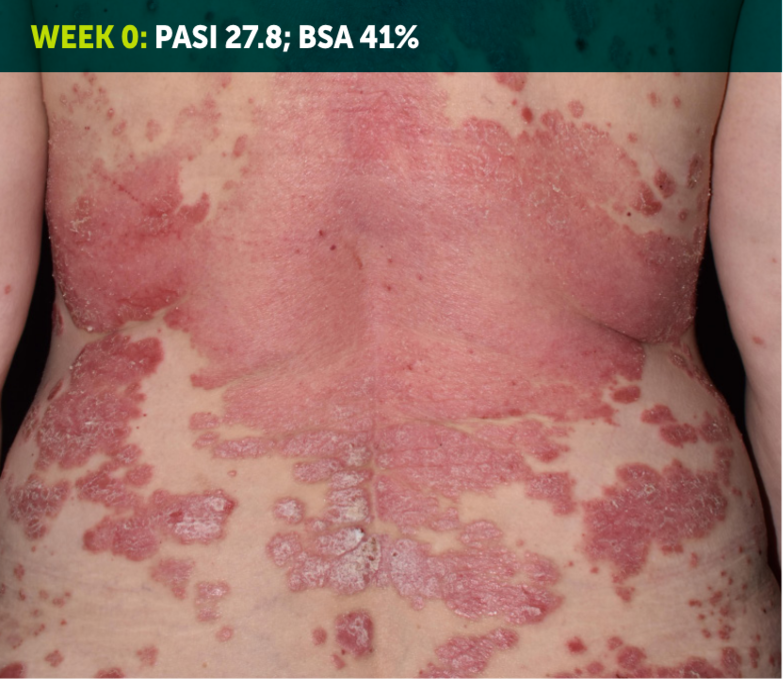
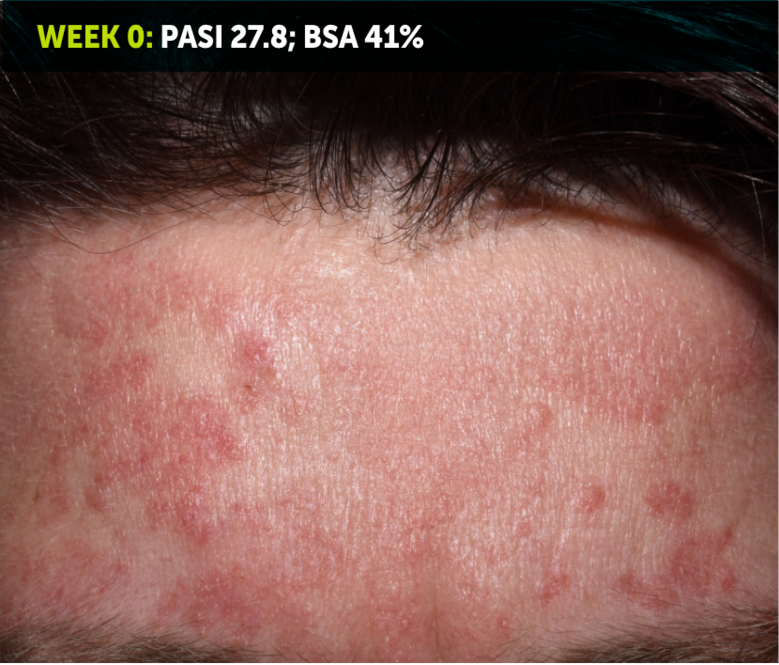
SEE TRANSFORMATIVE SKIN CLEARANCE3,4,7
Actual patient from the BE VIVID clinical trial who received a dose of 320 mg Q4W to Week 52.
The recommended dose for BIMZELX is 320 mg Q4W for 16 weeks, then Q8W thereafter. For patients ≥120 kg, a dose of 320 mg Q4W after Week 16 may be considered. The patient achieved PASI 90 at Week 16 (co-primary endpoint). Results are reflective of the average response in the trial patient population.
Actual patient from the BE RADIANT clinical trial who received a dose of 320 mg Q4W to Week 48.
The recommended dose for BIMZELX is 320 mg Q4W for 16 weeks, then Q8W thereafter. For patients ≥120 kg, a dose of 320 mg Q4W after Week 16 may be considered. The patient achieved PASI 100 at Week 16 (co-primary endpoint). Results are reflective of the average response in the trial patient population.
Actual patient from the BE RADIANT clinical trial who received a dose of 320 mg Q4W to Week 48.
The recommended dose for BIMZELX is 320 mg Q4W for 16 weeks, then Q8W thereafter. For patients ≥120 kg, a dose of 320 mg Q4W after Week 16 may be considered. The patient achieved PASI 100 at Week 16 (co-primary endpoint). Results are reflective of the average response in the trial patient population.
Actual patient from the BE RADIANT clinical trial who received a dose of 320 mg Q4W to Week 48.
The recommended dose for BIMZELX is 320 mg Q4W for 16 weeks, then Q8W thereafter. For patients ≥120 kg, a dose of 320 mg Q4W after Week 16 may be considered. The patient achieved PASI 100 at Week 16 (co-primary endpoint). Results are reflective of the average response in the trial patient population.
BSA=body surface area; PASI=Psoriasis Area and Severity Index; Q4W=every 4 weeks; Q8W=every 8 weeks.
BIMZELX PROVIDED CLEARANCE OF SCALP, PALMOPLANTAR, AND NAIL PSORIASIS7
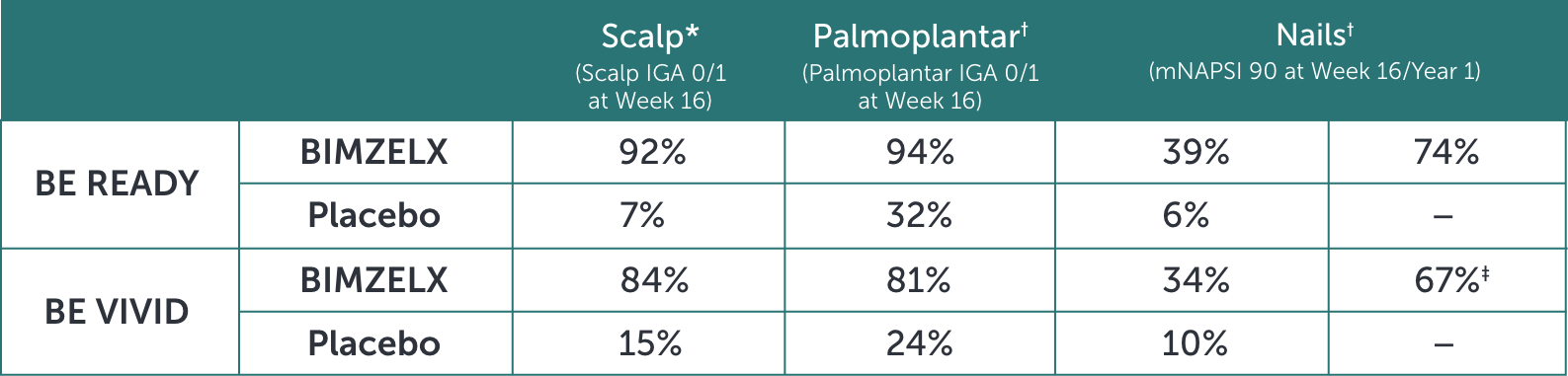
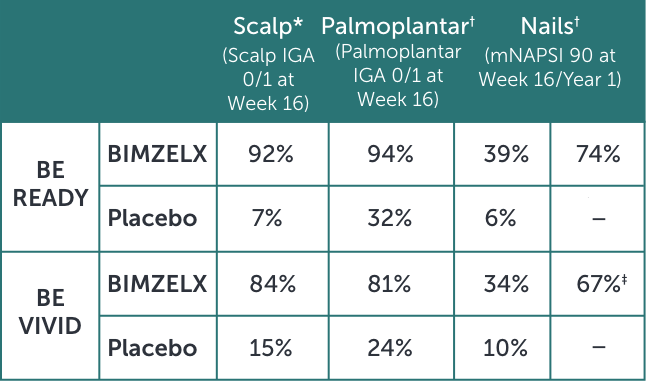
*Secondary endpoint adjusted for multiplicity P<0.001 for BIMZELX vs placebo in both studies.
†Other efficacy endpoint not adjusted for multiplicity.
‡Q4W dosing.
In BE READY 91%, 35%, and 60% of patients in the BIMZELX arm had scalp, palmoplantar, or nail involvement, respectively, at baseline. In the placebo arm, 91%, 45%, and 58% of patients had scalp, palmoplantar, or nail involvement, respectively, at baseline.
In BE VIVID 94%, 40%, and 60% of patients in the BIMZELX arm had scalp, palmoplantar, or nail involvement, respectively, at baseline. In the placebo arm, 88%, 40%, and 61% of patients had scalp, palmoplantar, or nail involvement, respectively, at baseline.
IGA=Investigator’s Global Assessment; mNAPSI=modified Nail Psoriasis Severity Index; Q4W=every 4 weeks.
BE READY: BIMZELX VS PLACEBO1,2
Trial Design: a pivotal, phase 3, multicenter, randomized, placebo-controlled, double-blind trial comparing BIMZELX vs placebo. Co-primary endpoints were the proportion of BIMZELX patients achieving PASI 90 and the proportion of patients achieving an IGA score of 0/1 at Week 16 vs placebo. Ranked secondary endpoints included the proportion of patients achieving PASI 100 and Week 16, PASI 75 and Week 4, and scalp IGA 0/1 at Week 16.
IGA 0/1=Investigator’s Global Assessment of clear or almost clear; PASI=Psoriasis Area and Severity Index.
BE RADIANT: BIMZELX VS COSENTYX®3
Trial Design: a phase 3b, randomized, double-blind, active comparator–controlled study comparing BIMZELX vs COSENTYX (300 mg weekly to Week 4, followed by every 4 weeks to Week 48). The primary endpoint was the percentage of BIMZELX patients achieving PASI 100 at Week 16 vs COSENTYX. Ranked secondary endpoints included the proportion of patients achieving PASI 75 at Week 4.
PASI 100=100% improvement from baseline in Psoriasis Area and Severity Index.
BE VIVID: BIMZELX VS STELARA® AND PLACEBO4
Trial Design: a pivotal, phase 3, multicenter, randomized, placebo-controlled, double-blind trial comparing BIMZELX vs placebo and STELARA (45 mg or 90 mg [baseline weight-dependent dosing] at Week 0 and 4, then every 12 weeks). Co-primary endpoints were the proportion of BIMZELX patients achieving PASI 90 and the proportion of patients with an IGA score of 0/1 at Week 16 vs placebo. Ranked secondary endpoints included the proportion of patients achieving PASI 90 and PASI 100 at Week 16 and PASI 75 at Week 4 vs STELARA. Efficacy computed using NRI.
IGA 0/1=Investigator’s Global Assessment of clear or almost clear skin; NRI=non-responder imputation; PASI 100=100% improvement from baseline in Psoriasis Area and Severity Index; PASI 90=≥90% improvement from baseline in Psoriasis Area and Severity Index; PASI 75=≥75% improvement from baseline in Psoriasis Area and Severity Index.
BE SURE: BIMZELX VS HUMIRA®5
Trial Design: a 56-week, phase 3, double-blind, randomized, active comparator–controlled trial comparing BIMZELX vs HUMIRA (40 mg every 2 weeks for 24 weeks). Co-primary endpoints were the proportion of BIMZELX patients achieving PASI 90 and the proportion of patients with an IGA score of 0/1 at Week 16 vs HUMIRA. The five ranked secondary efficacy endpoints were a PASI 100 response at Week 16, a PASI 75 response at Week 4, a PASI 100 response at Week 24, a PASI 90 response at Week 24, and an IGA score of 0/1 at Week 24.
IGA 0/1=Investigator’s Global Assessment of clear or almost clear skin; PASI 100=100% improvement from baseline in Psoriasis Area and Severity Index; PASI 90=≥90% improvement from baseline in Psoriasis Area and Severity Index; PASI 75=≥75% improvement from baseline in Psoriasis Area and Severity Index.
BE BRIGHT: OPEN-LABEL EXTENSION
Trial Design: Upon completion of BE READY, BE VIVID and BE SURE, patients could enroll in the BE BRIGHT OLE. Dose adjustments to BIMZELX Q4W or Q8W could occur at Week 56, and to BIMZELX Q8W at OLE Week 24 or OLE Week 48 and after based on PASI 90 response. The primary objective of the OLE was to assess the long-term safety and tolerability of BIMZELX in adult subjects with moderate to severe plaque psoriasis. Results shown include patients who received BIMZELX Q4W through Week 16 then Q8W thereafter (N=186).
OLE=open-label extension; PASI=Psoriasis Area and Severity Index; Q4W-every 4 weeks; Q8W=every 8 weeks.

WANT MORE INFORMATION ABOUT BIMZELX?